Abstract
Introduction: Acute myeloid leukemia (AML) is associated with poor outcomes in older and medically unfit patients, largely due to the severe toxicity associated with cytarabine treatment, which precludes the administration of effective cytarabine doses. BST-236 is a prodrug of cytarabine, inactive in its prodrug form and designed to deliver cytarabine to leukemia cells with reduced systemic toxicity, thus to enable delivery of high cytarabine doses to leukemia cells with relative sparing of normal tissues.
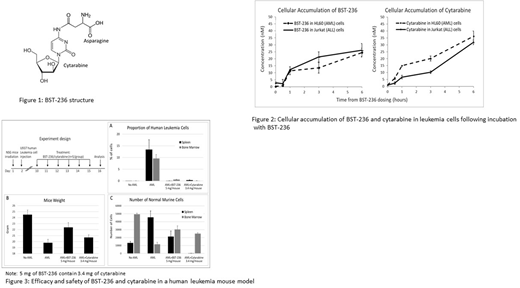
Results: BST-236 is a conjugate of cytarabine and asparagine (Figure 1). It was demonstrated that BST-236 is inactive as an intact prodrug and that its activity is exerted by release of cytarabine via non-enzymatic hydrolysis. Unlike free cytarabine, the bound cytarabine in BST-236 is not phosphorylated into its active metabolite Ara-CTP and it is protected by the asparagine residue from deamination into its inactive form Ara-U. In vitro studies demonstrate that BST-236 enters into leukemia cells, accompanied by cellular accumulation of free cytarabine, which is released from BST-236 (Figure 2). Like cytarabine, treatment with BST-236 result in induction of cell death of various leukemia cell lines via apoptosis, an activity which is dependent on the human equilibrative nucleoside transporter 1 (hENT1). The in vitro kinetics of BST-236-induced toxicity were found to be delayed compared to administration of free cytarabine, correlating with an observed delayed cellular availability of cytarabine.
In vivo studies in mice and dogs demonstrate that BST-236 concentrations in the plasma are dose-proportional, with a prodrug-typical profile and only ~5% of free cytarabine present in the plasma. The maximal tolerated dose of BST-236 was found to be several-fold higher than reported for cytarabine, with mainly hematological effects and no unexpected toxicities.
In vivo head-to-head studies in human leukemia mouse models with equimolar doses of cytarabine and BST-236 demonstrate similar efficacy of complete elimination of the leukemia cells in the bone marrow, spleen, and peripheral blood by both molecules (Figure 3A). However, while cytarabine treatment was associated with significant toxicity including weight loss, dramatic reduction in spleen size and number of mouse spleen cells, and delayed normal murine white blood cell recovery, equimolar BST-236 doses enabled spleen and BM recovery with minimal weight loss and no observed clinical signs (Figure 3B, 3C).
Summary: in vitro and in vivo studies demonstrate that BST-236 is a prodrug of cytarabine, which enables the delivery of cytarabine to target cells, resulting in elimination of the leukemia with reduced systemic toxicity compared to free cytarabine. The data also suggest that while the mechanism of cell death induced by BST-236 and cytarabine is similar, the observed differential kinetics of the delivery of cytarabine by BST-236 and its metabolism may explain its reduced systemic toxicity. Our nonclinical findings are in line with the clinical results of the BST-236 Phase 1/2 study (ASH 2017 abstract no 893, manuscript in preparation) and suggest that BST-236 may enable delivery of high cytarabine doses to older and medically-unfit patients who currently cannot benefit from an effective cytarabine therapy. This suggestion is to be confirmed by an ongoing Phase 2b study.
Tessler:Biosight: Employment. Gengrinovitch:Biosight: Employment. Ben Yakar:Biosight: Employment. Peled:Cellect Biotherapeutics Ltd: Consultancy. Flaishon:Biosight: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal